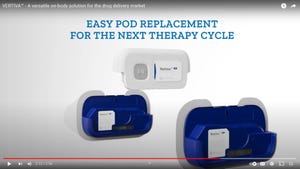
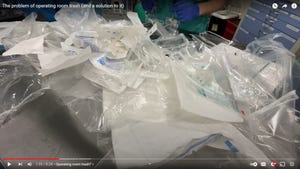
Pharmaceutical Packaging
Healthcare-GettyImages-843421738-ftd.jpg
Pharma & Medical
Healthcare Packaging News and Top IssuesHealthcare Packaging News and Top Issues
The future of pharma packaging podcast, UK’s non-profit initiative on recycling healthcare packaging, IoPP’s medical packaging ebook, packaging innovation workshop, more.
Sign up for the Packaging Digest News & Insights newsletter.