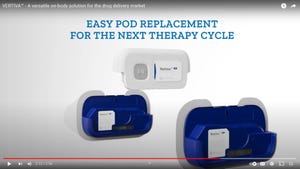
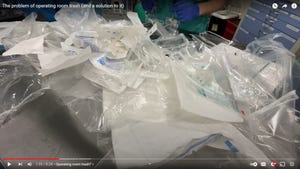
Pharmaceutical Packaging
Healthcare-GettyImages-843421738-ftd.jpg
Pharma & Medical
Healthcare Packaging News and Top IssuesHealthcare Packaging News and Top Issues
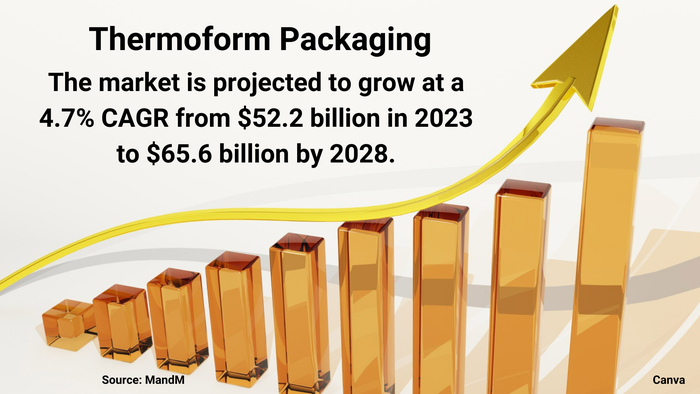
Top 10 in pharma packaging, sustainable packaging trends, track-and-trace panel discussion, meeting UDI label requirements, latest medical packaging forecast, future of pharma and med-device labeling, more.
Sign up for the Packaging Digest News & Insights newsletter.